By: Jacqueline J. Chan and Samantha Hong
Like all entities faced with navigating the challenges of resuming operations as the COVID-19 pandemic progresses, FDA too has had to adapt and adjust its inspection, investigation, and sampling operations to accommodate the changing landscape. On May 5, 2021, FDA issued its “Resiliency Roadmap for FDA Inspectional Oversight” report to detail its oversight activities over the last year after the onset of the pandemic—when FDA decided to pause most of its foreign and domestic inspections—and to provide the agency’s plan for inspectional activities moving forward.[1] Ultimately, FDA expects to continue implementing its COVID-19 inspection prioritization plan until the pandemic ends, which utilizes a risk-based approach with a focus on the highest priority inspections while balancing COVID-19 public health risks. As discussed further below, the ongoing plan consists of three tiers: (1) Tier 1: Mission Critical inspections, (2) Tier 2: Higher Priority inspections, and (3) Tier 3: Lower Priority inspections. To date, in implementing this tiered approach, FDA has prioritized the mission critical inspections along with application-based inspections and for-cause inspections, while postponing the majority of its routine surveillance-based inspections. The agency has also focused on alternative oversight tools, such as conducting remote inspections and leveraging information shared by foreign and state/local partners.
Moving forward, FDA intends to continue with its tiered inspection approach while also catching up on postponed surveillance inspections, as appropriate, depending on the pandemic’s trajectory. Notably, even in a best-case scenario where FDA resumes normal operations immediately, FDA estimates that it will only be able to complete 27% of the over 15,000 routine surveillance inspections remaining by the end of this fiscal year (September 30, 2021). In a worst-case scenario where the pandemic worsens, FDA expects to focus only on mission critical work while more heavily leveraging its alternative oversight tools. In all scenarios, it appears that industry can expect significantly reduced levels of routine inspections for the foreseeable future.
Key Takeaways from FDA’s Resiliency Roadmap for FDA Inspectional Oversight
Where FDA Started…
At the start of the pandemic, FDA focused solely on inspections that it deemed “mission-critical,” as determined on a case-by-case basis, concerning the following product categories:
- Product received breakthrough therapy or regenerative medicine advanced therapy designation.
- Product is used to treat a serious disease or medical condition and there is no substitute.
- Product requires follow-up due to recall, or there is evidence of serious adverse events or outbreaks of a foodborne illness.
- Product is related to FDA’s COVID-19 response (e.g., drug shortages).
In July 2020, FDA resumed “prioritized domestic inspections,” in addition to mission critical inspections, informed by the agency’s newly developed COVID-19 Advisory Rating system.[2] FDA’s prioritization decisions considered factors such as whether the inspection:
- Intends to follow-up on a previous violative inspection;
- Is needed to support a product approval decision where no other application deficiencies are known that would preclude approval;
- Is considered high-risk under statutory inspection frequency mandates; or
- Otherwise maximizes the use of limited inspectional resources to achieve the greatest public health impact during the COVID-19 pandemic.
FDA largely prioritized application-based inspections (i.e., pre-approval, pre-market, and pre-license inspections) and for-cause inspections (e.g., in response to consumer complaints or adverse event reports), while postponing the majority of its routine surveillance inspections mandated by various provisions of the Federal Food, Drug, and Cosmetic Act (FFDCA) (e.g., Food Safety and Modernization Act inspections). Indeed, FDA estimated that only 68 medical product application decisions, out of approximately 600 applications needing inspectional oversight, were delayed due to inspection delays. In contrast, FDA postponed tens of thousands of surveillance inspections and did not conduct any inspections of tobacco product establishments over the last year.
Where FDA is Headed… (We Think)
FDA intends to continue implementing the below inspection prioritization plan until the pandemic ends and travel restrictions are eased or lifted.
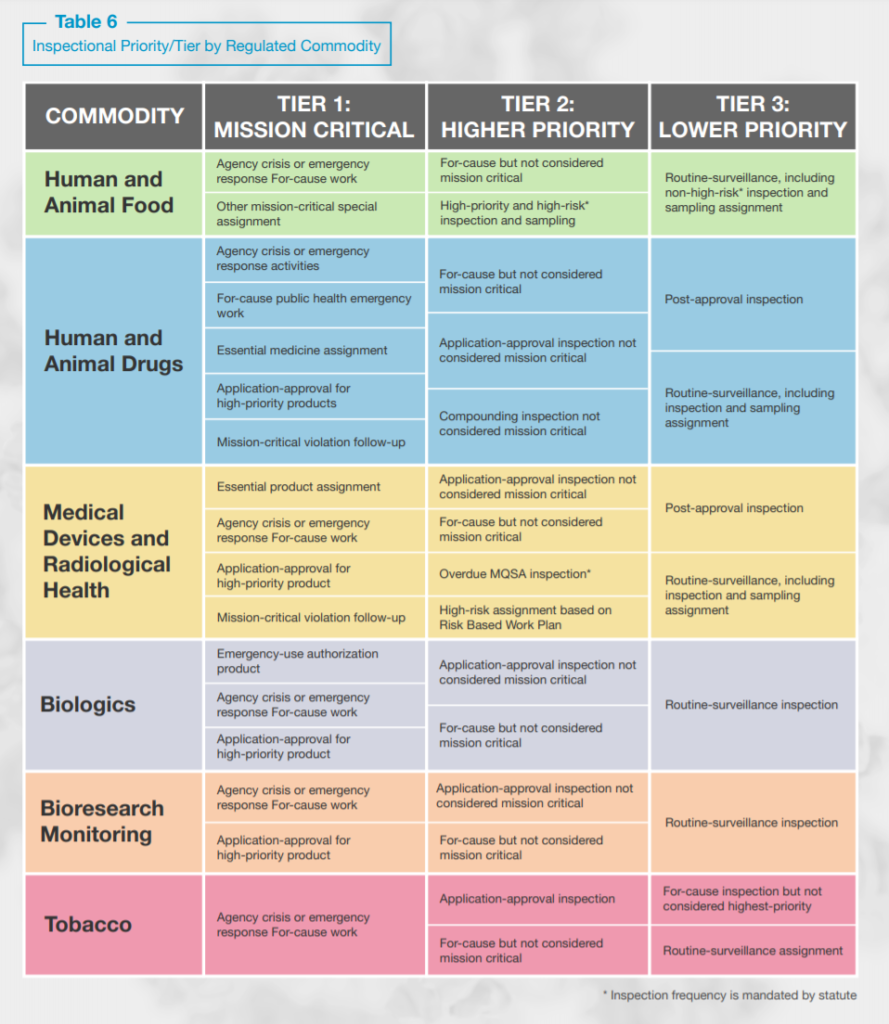
Consistent with FDA’s oversight activities over the last year FDA will focus on mission critical and higher priority inspections and, with respect to postponed routine surveillance inspections, FDA anticipates that “a longer interval between inspections will occur for lower-tiered inspection assignments as the agency adjusts to the impact of the COVID-19 pandemic.” As such, “postponed inspections will be prioritized based on risk and conducted over a longer period of time, ultimately increasing the amount of time between inspections of certain lower-risk facilities.”
By the numbers, FDA estimates that 15,514 domestic routine surveillance inspections remain to be conducted by September 2021, but that only a fraction of these routine surveillance inspections will be conducted:
- In a “best-case scenario” where FDA resumes standard operations immediately, FDA estimates that it will complete 27% of the remaining inspections.
- In a “base-case scenario” where FDA instead more gradually resumes standard operations by July 2021, it estimates that it will complete 14% of the remaining inspections.
- In a “worst-case scenario” where the pandemic continues and there is significant risk to FDA staff, FDA anticipates that it would focus on mission critical inspections and increase focus and continued reliance on alternative oversight tools such as:
- reviewing records and information requested from facilities or in lieu of certain drug and biological product inspections to support regulatory decisions and actions;[3]
- conducting remote inspections to oversee FDA Food Safety Modernization Act and Foreign Supplier Verification Program compliance;
- leveraging information shared by foreign regulatory partners, as well as state, local, tribal, and territorial regulatory partners;
- utilizing risk-based product sampling and analysis both domestically and at the border; and
- refusing entry of unsafe imported products.
Notably, FDA does not expect to conduct any foreign surveillance inspections by U.S.-based staff before September 2021 due to ongoing COVID-19 challenges (though some foreign inspections have occurred through FDA’s foreign offices).
FDA’s report leaves significant uncertainty regarding whether and when regulated industry will be subject to routine surveillance inspections over the next several months, though it seems that industry can expect to experience significantly reduced levels of such inspections for the foreseeable future. In addition, FDA has demonstrated increasing reliance on alternative oversight tools, which we can expect to continue so long as COVID-19 remains a public health threat. We will continue to monitor FDA’s inspection activities and updated guidance on the agency’s alternative oversight tools.
[1] See FDA News Release, Coronavirus (COVID-19) Update: FDA Outlines Inspection and Assessment Activities During Pandemic, Roadmap for Future State of Operations (May 5, 2021).
[2] FDA’s COVID-19 Advisory Rating system used system uses real-time data to assess the number of COVID-19 cases in a local area. See FDA Statement, Coronavirus (COVID-19) Update: FDA prepares for resumption of domestic inspections with new risk assessment system, July 10, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-prepares-resumption-domestic-inspections-new-risk-assessment-system.
[3] FDA is authorized to obtain such documents under FFDCA § 704(a)(4) (21 U.S.C. § 374(a)(4)). Also, for FDA guidance on its approach to voluntary remote evaluations of drug manufacturing and bioresearch monitoring facilities during the pandemic, see FDA Guidance for Industry, “Remote Interactive Evaluations of Drug Manufacturing and Bioresearch Monitoring Facilities During the COVID-19 Public Health Emergency,” April 2021, available at https://www.fda.gov/media/147582/download.
