By: Daniel Dwyer and T. Daniel Logan
As Shakespeare’s MacDuff said, in a slightly different context, “Confusion now hath made his masterpiece!” MacDuff’s exclamation aptly applies to the current state of state hemp law.
You’d think it would not be so: the 2018 Farm Bill, after all, built a national edifice of hemp rules: it defined hemp as Cannabis sativa L. (Cannabis) that contains not more than 0.3% THC including all derivatives, extracts, and cannabinoids, and established a USDA-supervised hemp production system under which a state can, if it wants, operate a program that is consistent with federal law. However, the Farm Bill said that federal law does not preempt state law so long as state hemp production rules are consistent with those of USDA. As a result, states are now enacting a variety of different requirements using the federal hemp production rules as their baseline.
For example, a number of states have enacted various types of testing, labeling, and registration requirements. The accompanying map uses different colors to highlight different types of hemp rules in different states.
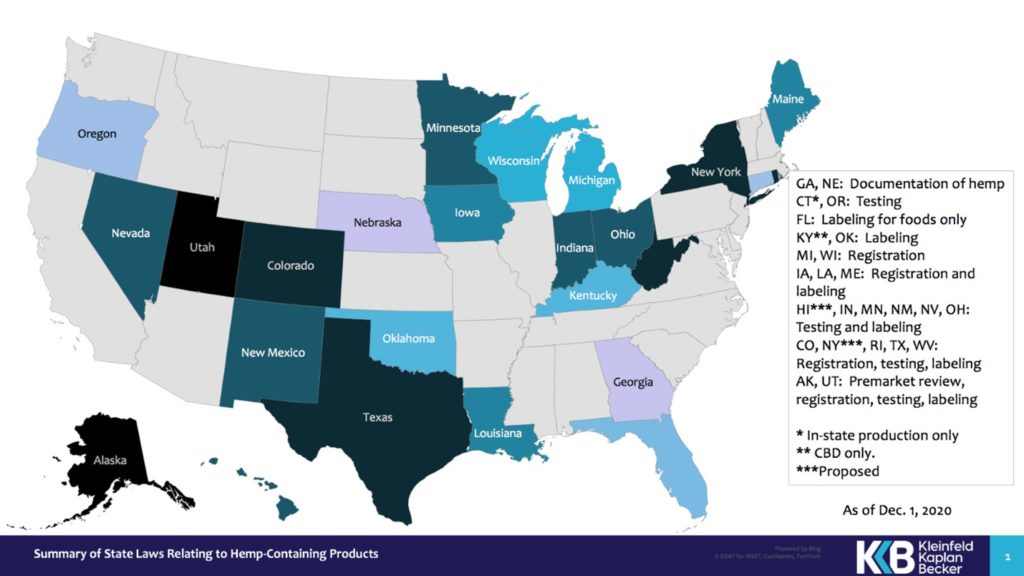
To explicate this rather complex picture:
- Two states have implemented testing requirements alone: Connecticut (in-state production only) and Oregon;
- Three states have labeling requirements alone: Florida (foods only), Kentucky (CBD only) and Oklahoma;
- Six states have requirements for both testing and labeling: Hawaii (proposed), Indiana, Minnesota, New Mexico, Nevada and Ohio;
- Three states have requirements for registration and labeling: Iowa, Louisiana and Maine;
- Five states have requirements for registration, testing and labeling: Colorado, New York (proposed), Rhode Island, Texas and West Virginia;
- Two states require some form of premarket review in addition to registration, testing and labeling: Alaska and Utah;
- Two states do not impose testing requirements but require documentation that Cannabis meets the definition of hemp: Georgia and Nebraska.
As you can see from the different colors on the map, even when taking a high-level look, there are a lot of differences between the states – a sort of patchwork quilt of hemp rules. The more you delve into the details, the more differences emerge.
In October 2020, New York State published proposed rules that set out a particularly comprehensive and well-thought-out effort to regulate hemp products. Though it’s hard to predict the future, this regulation, if published in final form, might be used as a model by other states. In any event, if a covered product is nationally marketed, it will need to comply with New York rules. The New York proposed rules apply broadly to consumable hemp products (such as foods, dietary supplements, and vape-type products) that are promoted as sources of cannabinoids (such as CBD) – except for cosmetics, which are not covered by the proposal.
New York’s proposed rule includes detailed manufacturing and formulation requirements, for example:
- Foods may not contain more than 25 mg cannabinoids per product. Supplements may not contain more than 3,000 mg per product.
- Registration is required for New York manufacturers and retailers.
- Manufacturers must follow good manufacturing practice, including complaint monitoring and recall plans.
- Cannabinoid hemp products, including foods, must be shelf-stable.
- Synthetic cannabinoids, alcohol, and tobacco may not be used.
- Certain product forms are not permitted, such as injectables, inhalers, or cigarettes made from hemp flowers.
- Electronic vaporization devices are permitted so long as they meet specific criteria (including no flavoring agents).
One particularly interesting aspect of the New York proposal is that it permits manufacturers to purchase and use intermediate hemp extract with THC levels up to 3% if it is only transported intrastate.
Another interesting aspect of this cover-all-the-bases proposal is that New York will require cannabinoid hemp products to be labeled with elements that go well beyond federal labeling requirements. Labels will need to include:
- Ingredient statements that are consistent with New York’s definition of “full spectrum” and “broad spectrum” hemp;
- THC and cannabinoid levels per serving and per package.;
- Expiration dates and batch numbers;
- Name of the hemp processor, and origin of the hemp;
- Specified warnings;
- A means for reporting serious adverse events;
- Dosing and instructions for use; and
- A bar or QR code linked to a downloadable certificate of analysis, with testing for:
- Pesticides
- Residual solvents
- Metals
- Microorganisms
- Mycotoxins
- THC.
You may be wondering whether it is possible for a product to comply with one set of labeling rules and be legal in all 50 states? Well … it will depend on the product and the formulation, in addition to the label. Other state laws are not necessarily consistent with the rules in New York. For example:
- Indiana requires the label to state that a product contains not more than 0.3% THC “including precursors,” whereas New York appears to permit at least some precursors to contain higher levels of THC.
- Louisiana does not permit CBD products to be sold to persons under 18.
- Minnesota requires testing laboratory information to be placed on the label, and prohibits structure-function claims.
- Rhode Island requires warnings different than those in New York.
- Utah limits hemp product forms more strictly than in New York.
- California and other jurisdictions purport to prohibit the sale of foods and dietary supplements containing hemp-derived CBD.
Products that are regulated as cosmetics are not covered by New York’s proposed rules, but would be covered by other states’ rules; for example, Texas, Utah and other states regulate cosmetics and impose registration, labeling, and other requirements. However, it is unclear what states consider to be “cosmetics” – and it is possible that, if a topical product were labeled with a benefit attributable to a cannabinoid, a state might decide that it’s not a cosmetic after all, but a drug ….
In light of the current exuberance of state regulation, a cautious marketer of hemp-containing products should conduct a careful, product-specific survey of state laws to ensure that a proposed product will be reasonably compliant in those states in which it may be sold or distributed. Moreover, this survey should be double-checked from time-to-time as state laws and regulations governing hemp have changed dramatically since 2018 and are still evolving.
In addition to state-law requirements, it is also important to remember that old saw about labels needing to be truthful and not misleading. Consumer plaintiffs have developed a fondness for class-action lawsuits over allegedly inaccurate claims about issues such as the levels of hemp ingredients, claims about the purity of products, and claims about the benefits of hemp ingredients – so making sure that claims match their scientific substantiation is important for hemp products (just as it is for all products).
Finally, we note that less than half of the US states have so far weighed in with specific rules for hemp products and it is anyone’s guess what the rest of them will do. This might be a good time for a beneficent group of experts to come up with a Uniform Model Hemp Product law!
